The Office of Inspector General (OIG) has made it abundantly clear in corporate integrity agreements (CIAs) over the past few years that it fully expects life sciences companies to monitor their activities to help ensure that company personnel are not violating company policies or procedures, not to mention federal health care program or Food and Drug Administration requirements. From direct observations to records reviews, the OIG has required a wide range of monitoring of both promotional and non-promotional activities.
If you would like to participate in a survey exploring the use of social media communications and the monitoring of such communications, please click here. Participants will receive a report examining the results of the survey.CIA monitoring requirements of promotional activities include observing product details by company employed sales representatives and speaker programs by contracted health care professionals (HCPs). Many CIAs also require monitoring of:
- Sales representatives’ emails and other electronic records
- Records of promotional materials provided by sales personnel to HCPs
- Message recall studies that capture the substance of sales representatives’ interactions with HCPs
To request a table comparing the monitoring requirements of recent CIAs, click here.
These CIA requirements demonstrate that the OIG expects companies to monitor what’s being communicated about their products through perhaps the most traditional of communication platforms – a field sales force. Given current CIA monitoring requirements, is there any reason to believe that future CIAs will not require the monitoring of social media and Internet communications, especially as life science companies increase their use of these platforms?
The FDA has already established a history of enforcement letters against life sciences companies communicating promotionally via social media and the Internet. Below are descriptions of three recent FDA letters involving social media/Internet advertising communications and predictions of possible monitoring requirements if and when the OIG enters into a CIA with a company alleged to have illegally promoted its products by means of such communications.
Possible monitoring requirement: Conduct search engine queries for sponsored links
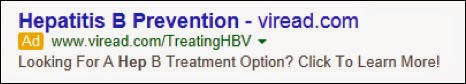
In June 2014, the FDA issued an untitled letter to Gilead Sciences in connection with its Google-sponsored link, which is reprinted below.
The letter stated this sponsored link “misleadingly suggests that Viread is safe and effective for use in the prevention of hepatitis B.” According to the drug’s approved labeling, Viread is indicated for the treatment of, but not the prevention of, hepatitis B.
The FDA concluded that “the sponsored link provides evidence that Viread is intended for a new use for which it lacks approval, and for which its labeling does not provide adequate directions for use, which renders Viread misbranded or otherwise makes its distribution violative.” This is post-Caronia FDA code for the conclusion that Gilead engaged in off-label promotion of Viread.
Possible monitoring requirement: Review claims on social media pages
In March 2014, the FDA issued an untitled letter to Institut Biochimique SA and its U.S. agent Akrimax Pharmaceuticals challenging communications on the company’s Facebook page as false and misleading. The FDA stated:
“While the Facebook webpage makes suggestions regarding the use of Tirosint in patients with hypothyroidism, it fails to convey that Tirosint is not indicated for transient hypothyroidism during the recovery phase of subacute thyroiditis.”
In fact, the product’s labeling expressly states that the drug is not indicated for such transient hypothyroidism. As a result, the FDA concluded that the Facebook page misbranded Tirosint.
While it’s clear that a company is responsible for and can be held liable for misleading and/or off-label communications on a company Facebook page, it’s less clear what responsibility a company may have for such communications on an employee’s Facebook page. Therefore, a company’s social media policy should unambiguously address what employees are allowed to communicate regarding a company’s products via social media platforms.
Possible monitoring requirement: Review “likes” on social media pages
In June 2014, the FDA issued a warning letter to Zarbee’s Inc. regarding, among other things, instances in which the company “liked” testimonials made by customers on the company’s Facebook page. For example, Zarbee’s “liked” a comment stating that the company’s cough syrup relieved the symptoms of a customer who had been “battling either bronchitis or pneumonia.”
While not a “mainstream” pharmaceutical company, the FDA stated in its warning letter that Zarbee’s introduced a new drug into interstate commerce through various claims on its Facebook page which demonstrated that the company’s cough syrup products “are intended for use in the cure, mitigation, treatment or prevention of disease.” It’s not difficult to imagine other companies “liking” statements on a social media page that relate to an unapproved use for an approved product and the FDA again seeing that response as off-label promotion.
These and other FDA enforcement letters show that the agency continues its surveillance of social media and Internet advertising communications. If the FDA is watching social media communications for off-label and other statements that misbrand a product, one should assume that potential whistleblowers are watching too. And where there’s a whistleblower, it’s reasonable to anticipate that the OIG will not be far behind with a CIA requiring a company to monitor its social media communications.



 Brian Dahl is the Principal at Dahl Compliance Consulting LLC. His consulting practice focuses on assisting life sciences companies with their corporate compliance needs. He is the architect of the corporate compliance programs at two top-tier pharmaceutical companies – Teva Pharmaceuticals and Takeda Pharmaceuticals. As a consultant, he has built the compliance programs at two startup companies that recently launched their first products. Brian brings that real-world experience to the service of clients who are developing, implementing, or evaluating the effectiveness of their corporate compliance programs. Brian spent six years as the Compliance Director at Teva, where he built the company’s compliance program from the ground up while leading all aspects of the company’s compliance efforts across multiple branded divisions. Brian’s career in pharmaceutical corporate compliance began at Takeda in 2001, six months before the government’s seminal settlement with TAP Pharmaceuticals. Prior to becoming a pharmaceutical compliance professional, Brian practiced health law at the law firm of Baker & Daniels. He began his legal career practicing advertising law in Washington, D.C., first at the Federal Trade Commission and later at the law firm of Collier, Shannon, Rill & Scott. Brian received his J.D. from the University of Iowa College of Law and his Master of Health Administration degree from the College of Public Health at the University of Iowa. You can reach Brian at 847-800-1753 or at DahlComplianceConsulting@gmail.com.
Brian Dahl is the Principal at Dahl Compliance Consulting LLC. His consulting practice focuses on assisting life sciences companies with their corporate compliance needs. He is the architect of the corporate compliance programs at two top-tier pharmaceutical companies – Teva Pharmaceuticals and Takeda Pharmaceuticals. As a consultant, he has built the compliance programs at two startup companies that recently launched their first products. Brian brings that real-world experience to the service of clients who are developing, implementing, or evaluating the effectiveness of their corporate compliance programs. Brian spent six years as the Compliance Director at Teva, where he built the company’s compliance program from the ground up while leading all aspects of the company’s compliance efforts across multiple branded divisions. Brian’s career in pharmaceutical corporate compliance began at Takeda in 2001, six months before the government’s seminal settlement with TAP Pharmaceuticals. Prior to becoming a pharmaceutical compliance professional, Brian practiced health law at the law firm of Baker & Daniels. He began his legal career practicing advertising law in Washington, D.C., first at the Federal Trade Commission and later at the law firm of Collier, Shannon, Rill & Scott. Brian received his J.D. from the University of Iowa College of Law and his Master of Health Administration degree from the College of Public Health at the University of Iowa. You can reach Brian at 847-800-1753 or at DahlComplianceConsulting@gmail.com.







